Chicago WIHS History
In 2012, Chicago WIHS moved to a dedicated facility to develop a larger clinic space and unique laboratory and repository. Chicago WIHS maintains a cohort of approximately 194 HIV-infected and 75 uninfected at-risk women in order to investigate clinical manifestations, disease progression and outcomes of HIV infection in women. 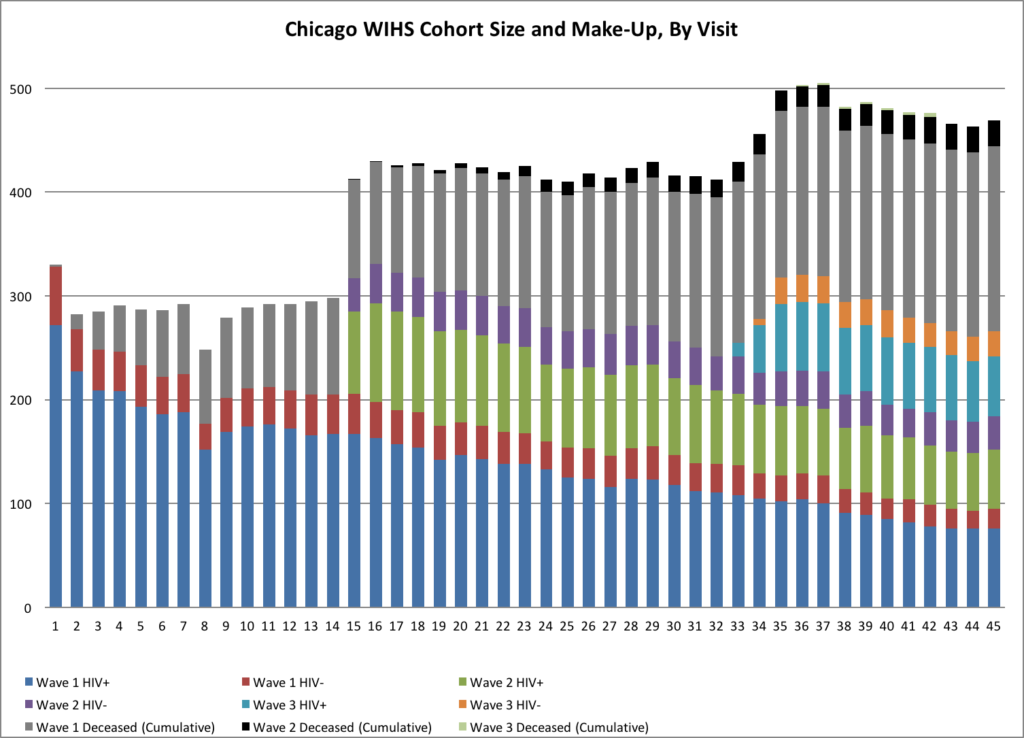
Our Chicago WIHS clinic is also the home of the Chicago WIHS processing laboratory and local repository, which is a Biological Safety Level 2 laboratory. We are certified for cryopreservation of human peripheral blood mononucleocytes (PBMCs) through the Duke Human Vaccine Immunological Quality Assessment Laboratory (IQA) cryopreservation program. This program certifies laboratories quarterly on their proficiency in freezing PBMCs and Chicago WIHS has remained in good standing in this program since opening.
The laboratory at Chicago WIHS contains the necessary equipment to perform a wide range of ELISA assays. The laboratory is also equipped to perform RNA and DNA extractions from a variety of cellular types, most recently DNA from microbiota. In addition to RNA & DNA extraction facilities the laboratory is equipped with an ABI geneamp 9700 thermal cycler for the amplification of DNA and RNA for further quantification or analysis. The Chicago WIHS processing laboratory and local repository has two liquid nitrogen freezers (LN2) and nine – 80°C freezers which contain cryopreserved PBMCs and serum, plasma, urine, CVL and pellet samples respectively. The laboratory has a total of 253,125 aliquots of PBMCs, pellets, urine, serum, plasma and CVLs stored locally. The laboratory uses the Laboratory Data Management System (LDMS) designed by Frontier Science Foundation (Buffalo, NY) to order aliquots, generate labels, and track and ship individual samples. In addition to our WIHS visits, we collaborate with various local investigators and conduct several local substudies.
To learn more about collaborating with WIHS, please see here.
