History of the Cook County
Clinical Research Site
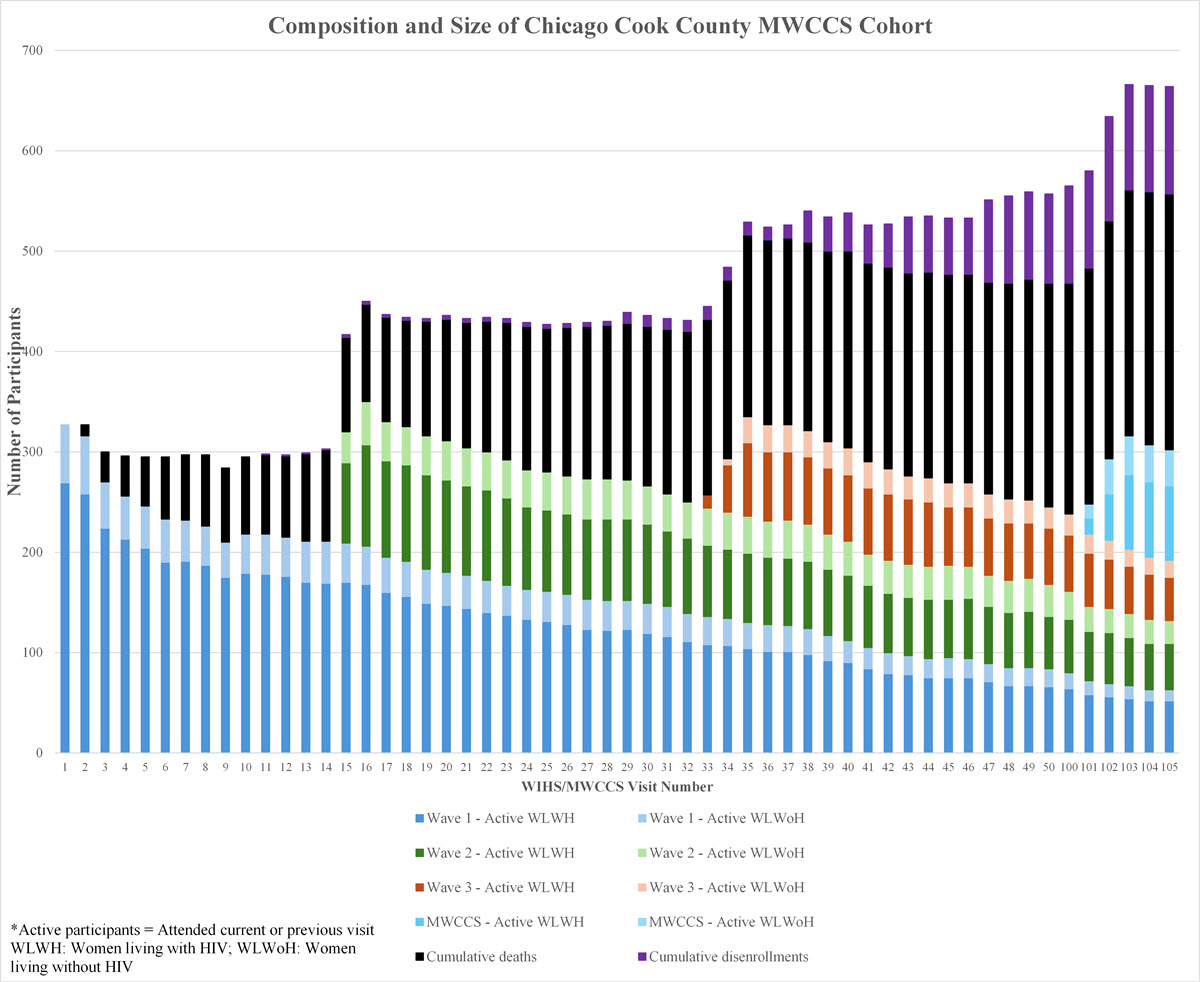
The Cook County Clinical Research Site (CC-CRS) began enrolling women living with and at risk for HIV in 1993 as a Women’s Interagency HIV Study (WIHS) site. Several national WIHS sites were funded by the National Institutes of Health at that time and later expanded to include sites in the Southern US in response to the growing realization that women with and at risk for HIV were under-represented and their unique manifestations and risk factors were not being adequately addressed. There were several waves of enrollment to reflect the changing epidemiology of US women with HIV (see Figure).
Dr. Mardge Cohen, who founded the Women and Children’s HIV Program at Cook County Hospital, led a successful city-wide application and joined sites in Los Angeles, Washington DC, San Francisco, Bronx, and Brooklyn. The Cook County site was unique because the research program was led by and integrated within a public health and hospital system. Dr. Cohen submitted the initial Chicago WIHS grant and became the site’s first Principal Investigator (PI). She forged a partnership with Kathleen Weber to develop and direct the new and now highly successful research program. Ms. Weber has served as Project Director of the Cook County site since its inception in 1994. Dr. Audrey French joined the WIHS in 1997 as an additional PI and in 2024, Dr. Ryan Ross joined the team as the site’s third PI. Currently, the CC-CRS includes a primary site at 2225 W. Harrison (administrative, clinical, and laboratory/repository), a clinical subsite at Rush University led by Dr. Beverly Sha, and several collaborating investigators across multiple institutions and departments (see organizational chart and investigator profiles for more detail).
In 2019, the WIHS combined with the Multicenter AIDS Cohort Study (MACS), a similar study of men initiated in 1993, to form the largest mixed-sex longitudinal HIV cohort of HIV in the country (MACS/WIHS Combined Cohort Study) with a shift in emphasis on the comorbidities affecting those aging with HIV. During the past three decades, the CC-CRS has remained highly successful with a sustained record of excellence in recruitment, retention, data quality, and publication record. Our site has contributed important work describing behavioral health and neurocognitive disorders, gynecologic manifestations, causes of death, the impact of co-infections such as HCV and HPV, sleep and circadian function, social determinants and neighborhood stressors, the effects of Incarceration, pathogenesis, and immune-aging (see Publications).
We are grateful for the continued confidence of our NIH colleagues in funding the CC-CRS, and our dedicated, resourceful, and compassionate staff, many of whom have been with CC-CRS for several decades. Most of all, we are grateful for the unwavering commitment of our dedicated cohort of women who have become invaluable partners in our effort to fully understand the impact of HIV in the lives of women. Without their steadfast participation – advocacy, input, courage, and time – the many ongoing advances in improving the care and quality of life for women aging with HIV would not be possible.